Are you or a loved one living with a small to mid-sized abdominal aortic aneurysm (AAA)?
The stAAAble Clinical Study may be right for you.
Nectero Medical is conducting a clinical trial for the Nectero Endovascular Stabilization Treatment (Nectero EAST®) System in small to mid-sized abdominal aortic aneurysms (AAA). The Nectero EAST System is an investigational novel treatment that may slow AAA growth; may reduce the risk of rupture/bursting of smaller AAAs and may prevent or significantly delay the need for major endovascular repair (EVAR) or open surgical repair.
Are you eligibleKey Eligibility Requirements for the stAAAble study
To participate in the study, patients must be between 21 and 85 years of age and diagnosed with a small to mid-sized AAA. Females must be of non-childbearing potential (menopause or sterilization).
Female AAA size
3.5-4.5cm
Female AAA size
3.5-4.5cm
Male AAA size
3.5-5.0cm
Male AAA size
3.5-5.0cm

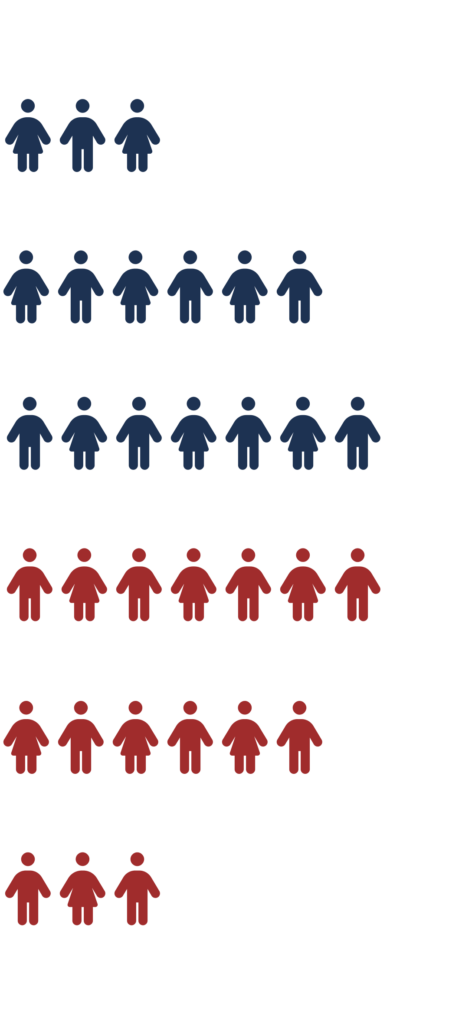
If you qualify to participate in the stAAAble study, you will be randomly assigned to one of 2 groups
All enrolled patients are likely to benefit through study-related medical examinations to monitor progress as well as increased awareness of their condition.
50%
of participants will receive the investigational study treatment
50%
of participants will be in the non-treatment group
Choosing to join a clinical research study is a significant and personal decision. Before you agree to participate, the study team will explain all the details to you and clearly outline the study’s potential benefits and risks. You will have the opportunity to ask questions and are encouraged to discuss with your doctor if study participation is the right choice for you.
More information about the stAAAble study can be found at www.clinicaltrials.gov (NCT06001918).
If you think that you or your loved one may be eligible for the study, please:
1
Search the map below for a study site near you
Contact information will be provided
2
Contact the site and let them know you would like to be considered for the stAAAble study of the Nectero EAST System in small to mid-sized AAA
Please remember to leave your name and best way to contact you
3
A study member from the site will respond to answer your study-related questions and request additional information if appropriate
Please be sure to have the following information ready:
- Insurance information
- List of current medications
- Size of aneurysm
• Insurance information
• List of current medications
• Size of aneurysm
Participating clinical study site locations
This study is being conducted at various institutions around the country. If you or your loved one are living with a small to mid-sized AAA, meet study key eligibility criteria and are interested in learning more about the stAAAble study, please use the interactive map to find the contact information for the study location closest to you.
Contact:
Principal Investigator:
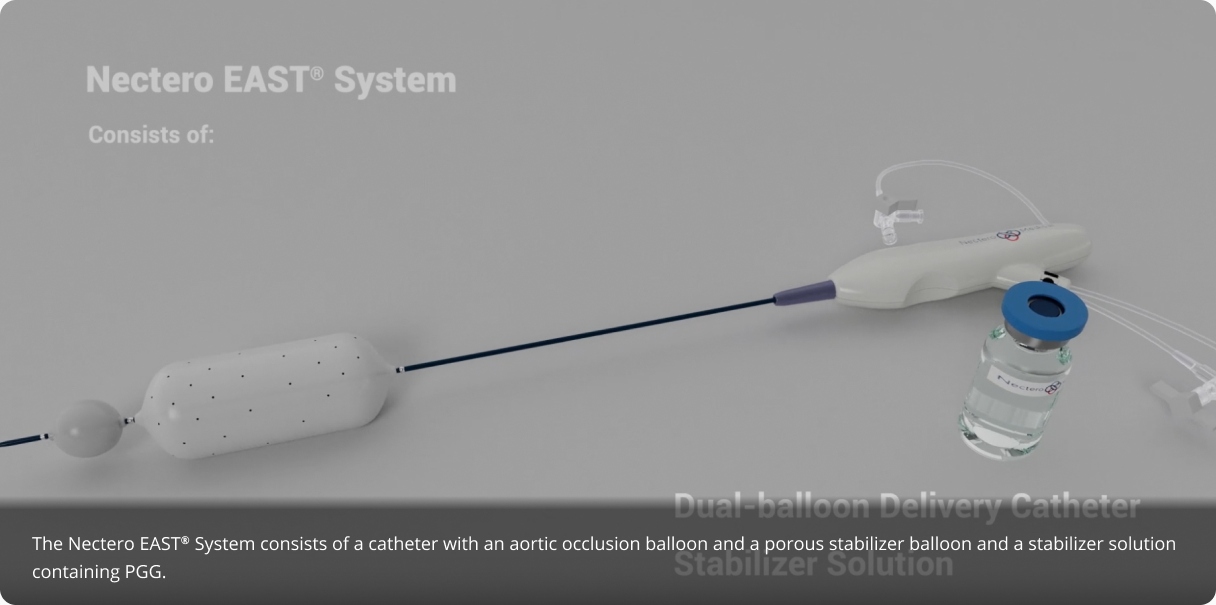
About the Nectero EAST® System
The investigational Nectero Endovascular Aneurysm Stabilization Treatment (Nectero
EAST®) System utilizes a catheter, a thin, long, tube-like device with inflatable
balloons at the end. The catheter is introduced through a small cut in the groin and is used to
deliver the investigational medicine directly to the site of the aneurysm via microscopic holes
in the balloon.
The procedure is performed using sedation, and is a one-time
treatment that may have a lasting effect. This minimally invasive procedure takes less than an
hour to complete. Once inside the aneurysm, the investigational drug may act to strengthen the
artery wall and prevent further weakening.
The goal for this investigational therapy is to allow earlier intervention or treatment of small to mid-sized AAA. If successful, the treatment may potentially:
Slow or prevent aneurysms from growing
Lower risk of aneurysm rupture/bursting
Prevent or significantly delay the need for major endovascular repair (EVAR) or open surgical repair
Understanding AAA
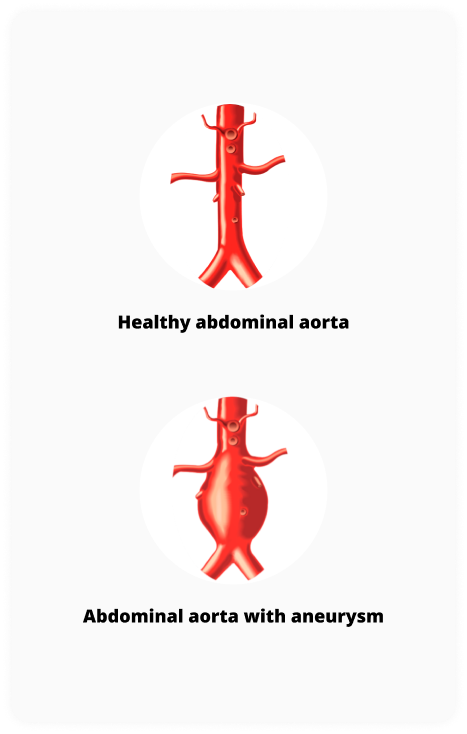
The aorta is the largest blood vessel in the body, carrying blood away from the heart to the rest of the body. The abdominal aorta is the portion located in the abdomen or stomach area. The normal width of the aorta is approximately 2 centimeters (cm), similar to a garden hose.
AAA stands for Abdominal Aortic Aneurysm, which is a bulge or ballooning in a weakened part of the abdominal aorta. Due to age, genetic conditions, disease or trauma, the wall of the aorta can become weak, causing the aortic wall to bulge or stretch, which can cause the aortic wall to weaken further. The larger the aneurysm grows, the greater the risk of rupture.
AAAs are common and are the 14th leading cause of death in the United States. Each year in the United States, AAA rupture causes 4,500 deaths, with an additional 1,400 deaths resulting from the ~40,000 repair procedures performed to prevent rupture (reference).
If an AAA bursts, it can cause severe internal bleeding. A ruptured AAA is life-threatening and needs urgent medical care.
Current treatment options for AAA in the US
Treatment options for AAA are primarily based on aneurysm size.
For most patients with small to mid-sized AAA, there is currently no proven treatment
- AAA that is 3.5 to 5.0cm (for men) or 3.5 to 4.5cm (for women) is considered small to mid-sized
- Physicians will place the patient under surveillance, or “watch and wait” to track the size and growth of the aneurysm
- Physicians may monitor and measure the AAA with regular ultrasound or computed tomography angiography (CTA) scans
Larger aneurysms are treated with endovascular repair (EVAR) or surgery
- AAA larger than 5.5cm for men or larger than 5.0cm for women is considered large