Abdominal aortic aneurysms (AAAs) occur in nearly

of the population1
In the US

4500
deaths per year are attributed to AAA ruptures2
An additional
1400
deaths annually result from attempted AAA repair2

- Prevalence and Trends of the Abdominal Aortic Aneurysms Epidemic in General Population – A Meta-Analysis, PLoS One, 2013; Li, et al. 8(12): e81260
- The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001–2004. McPhee JT, Hill JS, Eslami MH. J Vasc Surg. 2007;45:891–9
No Proven Options for Small- to Mid-Sized AAAs
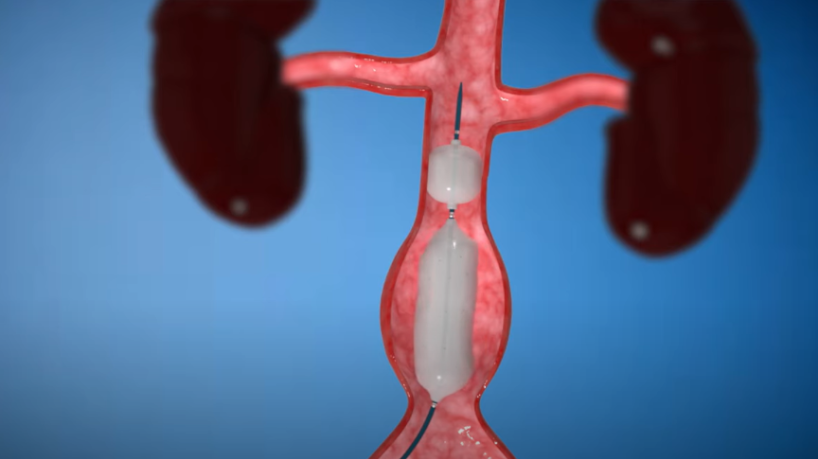
Nectero EAST® System
The Nectero Endovascular Aneurysm Stabilization Treatment (Nectero EAST®) system is a minimally invasive technology currently undergoing clinical trials. If successful, it may offer patients with smaller AAAs a first approved therapeutic option beyond surveillance.
- Comprised of a dual-balloon delivery catheter and a stabilizer mixture containing pentagalloyl glucose (PGG)
- Delivers drug locally into the aneurysmal wall
- PGG binds to elastin and collagen to potentially strengthen the aortic vessel wall and reduce the risk of further degradation
- Procedure takes <1 hour to complete
- No permanent implant is left behind
- Future interventions are not precluded
Pre-clinical Data
Enzymatic degradation of elastin and collagen results in weakening of the aortic wall and gradual growth and expansion of AAA. Localized treatment that prevents the enzymatic degradation from occurring has the potential to slow or stop an aneurysm from growing and rupturing.
In variety of animal studies, PGG has demonstrated the potential to:
- Strengthen weakened tissue
- Sterically hinder enzymatic degradation
- Inhibit growth of aneurysms
- Reduce burst risk

Pentagalloyl Glucose (PGG)
Clinical Data
stAAAble Study
In July 2023 the US Food and Drug Administration (FDA) granted Investigational New Drug (IND) clearance for the company to initiate a prospective, multi-center, randomized clinical trial to evaluate the safety and efficacy of the Nectero Endovascular Aneurysm Stabilization Treatment (Nectero EAST®) System in patients with infrarenal abdominal aortic aneurysms (AAAs), maximum diameter 3.5 – 5.0cm. Learn more
Breakthrough Designation
In October 2023, Nectero EAST® System was granted Breakthrough Therapy designation to treat patients with infrarenal AAAs, maximum diameter 3.5 – 5.0cm.
- Designation intended to expedite the development and review of drugs or combination products to treat a serious condition, and for which preliminary clinical evidence indicates that the drug or combination product may provide a substantial improvement on clinically significant endpoint(s) over available therapy
- Intensive FDA guidance on an efficient drug development program, including FDA’s organizational commitment to involve senior managers
- More frequent meetings with the FDA to ensure appropriate data collection in support of drug approval
Expanded Access
Nectero Medical encourages participation in the stAAAble trial as the best way for patients to access investigational products prior to regulatory approval. When it is not possible for a patient to participate in a clinical trial and all other available medical options have been exhausted, physicians may seek expanded access to an investigational product. Expanded Access Policy
FIH/Phase I Study
- A global, prospective, multi-center study of the Nectero EAST® System to treat small to mid-sized AAAs was performed outside the U.S.
- Primary endpoints were technical success and safety (major adverse events at 30 days)
- Secondary endpoint was growth stabilization, defined as freedom from aneurysm sac enlargement (diameter increase >5 mm per year or volume increase of > 10% per year)
- Safety and preliminary efficacy results on the initial 20 patients (19 male) were recently published in the Journal of Vascular Surgery with the following results:
- 100% procedural success
- 30-day primary safety data indicated the procedure was safe
- Efficacy data on the initial 11 patients were promising and supported proceeding with further studies
Key Publications
Preclinical
- Abdominal aortic aneurysm: A comprehensive review. Aggarwal S, Qamar A, Sharma V,, Sharma A. Exp Clin Cardiol 2011; Spring 16(1): 11-15.
- Elastin stabilization for treatment of abdominal aortic aneurysms. Isenburg J, Simionescu D, Starcher B, Vyavahare N. Circulation 2007; 115: 1729-01737.
- Chemical stabilization of the extracellular matrix attenuates growth of experimentally induced abdominal aorta aneurysms in a large animal model. Simionescu D, Casco M, Turner J, Rierson N, Yue J, Ning K. JVS Vasc Sci. 2020 Apr 23; 1:69-80.
- Binding of pentagalloyl glucose to aortic wall proteins: insights from peptide mapping and simulated docking studies. Simionescu D, Tharayil N, Leonard E, Carlyle W, Schwarz A, Ning K, Carsten C, Carrillo Garcia J, Carter A, Owens C, Simionescu A. Bioengineering August 2023; 10(8), 936.
Clinical
- A pilot study to evaluate a novel localized treatment to stabilize small- to medium-sized infrarenal abdominal aortic aneurysms. Stephen W. K. Cheng, et. al. J Vasc Surg. 2023 Oct;78(4):936. doi: 10.1016/j.jvs.2023.06.007.